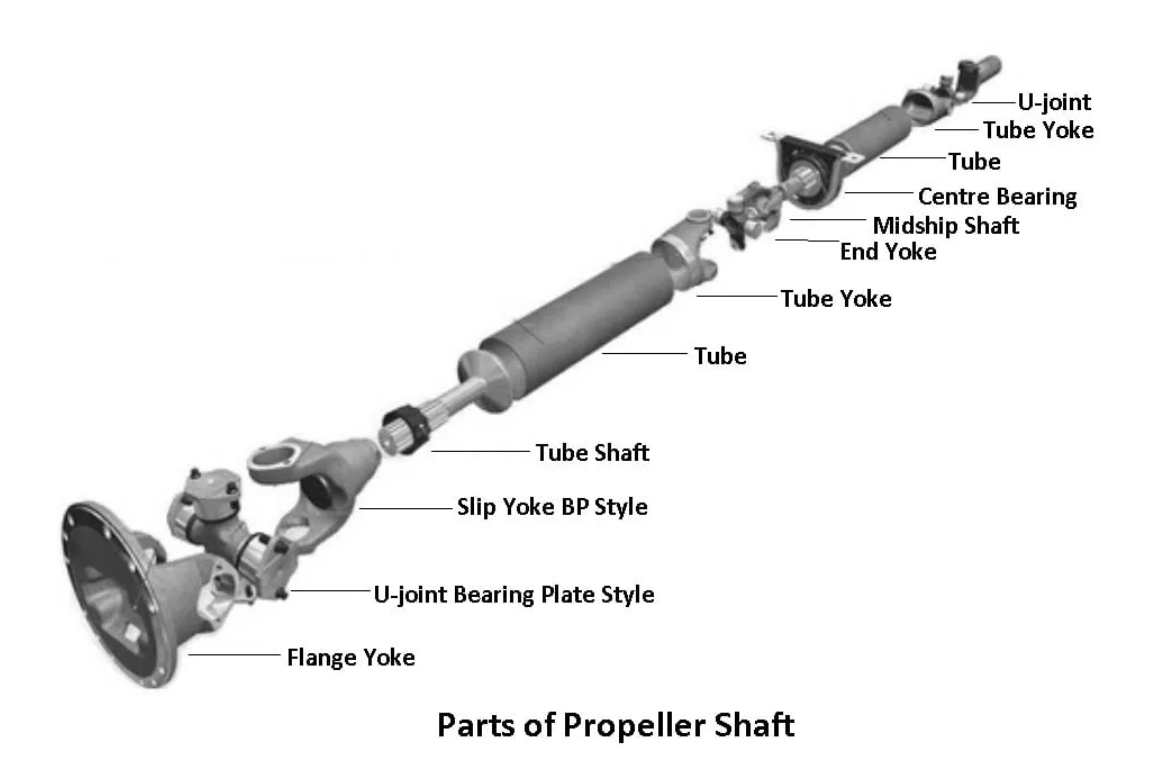
what is catalytic converter
A catalytic converter is a device used in the exhaust system of internal combustion engines, primarily in vehicles, to reduce the harmful emissions produced during the combustion process. It works by facilitating chemical reactions that convert pollutants and harmful gases, such as nitrogen oxides (NOx), carbon monoxide (CO), and unburned hydrocarbons, into less harmful substances like nitrogen, carbon dioxide, and water vapor.
The catalytic converter contains a catalyst, typically made of precious metals like platinum, palladium, and rhodium, that acts as a facilitator for these chemical reactions. The exhaust gases flow through a honeycomb-like structure coated with the catalyst material. As the gases pass over the catalyst, the chemical reactions occur, transforming the pollutants into less harmful compounds.
The catalytic converter plays a crucial role in reducing air pollution and meeting emissions standards set by regulatory authorities. It has become a standard component in most vehicles worldwide, contributing to improved air quality and reduced environmental impact from vehicle emissions.
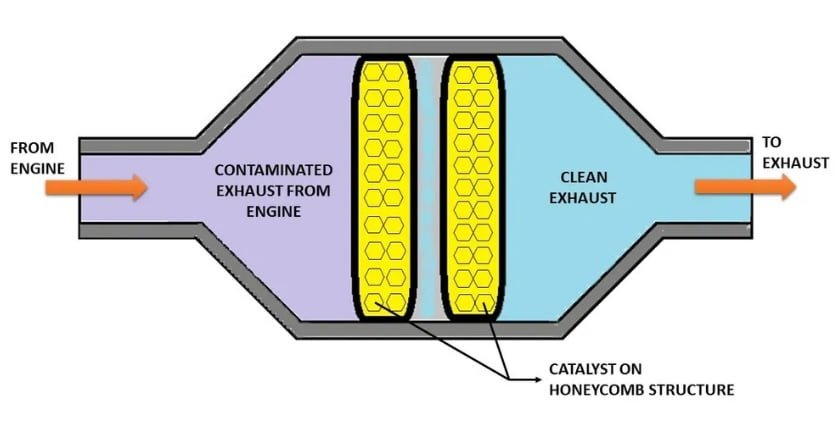
Construction of catalytic converter
A catalytic converter consists of several key components designed to facilitate the chemical reactions that convert harmful exhaust gases into less harmful substances. The construction of a typical catalytic converter involves the following main parts:
- Casing: The outer shell of the catalytic converter is usually made of stainless steel or another heat-resistant material. It provides protection to the internal components and ensures that the high temperatures generated during the catalytic reactions are contained within the unit.
- Catalyst Support: Inside the casing, there is a substrate or support material that holds the catalyst. This substrate is often a ceramic or metallic honeycomb structure with a large surface area. The honeycomb design provides ample contact area for the exhaust gases to interact with the catalyst.
- Catalyst Coating: The catalyst, composed of precious metals like platinum, palladium, and rhodium, is applied as a thin washcoat onto the substrate. These metals act as the catalysts for the chemical reactions that occur within the converter. The washcoat enhances the surface area available for reactions to take place.
- Oxygen Sensors: Modern catalytic converters often have oxygen sensors positioned before and after the converter. These sensors monitor the oxygen levels in the exhaust gases, providing feedback to the engine control unit (ECU) to optimize the air-fuel mixture for efficient catalytic conversion.
- Heat Shield: Catalytic converters generate substantial heat during their operation. A heat shield made of insulating materials is often placed around the converter to prevent excessive heat from affecting surrounding components and to protect against potential heat-related damage.
The process of catalytic conversion involves several chemical reactions:
- Oxidation of CO: Carbon monoxide reacts with oxygen to form carbon dioxide.
- Reduction of NOx: Nitrogen oxides are reduced to nitrogen and oxygen.
- Oxidation of HC: Unburned hydrocarbons are oxidized into carbon dioxide and water.
These reactions occur on the surface of the catalyst when exhaust gases pass through the substrate. The catalyst provides a surface where the reactions can take place at lower temperatures than would otherwise be required.
Overall, the construction of a catalytic converter is carefully designed to maximize the efficiency of these chemical reactions, thereby reducing the emission of harmful pollutants from the vehicle’s exhaust gases.
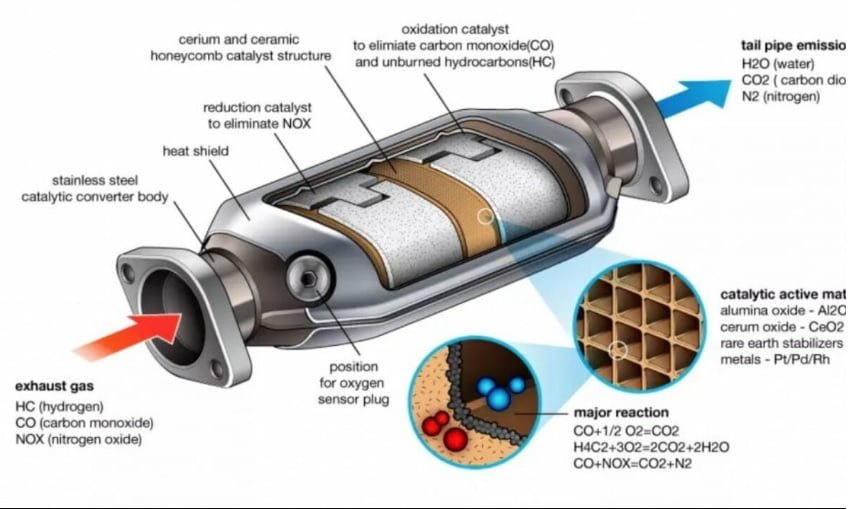
how does catalytic converter works
A catalytic converter works by facilitating chemical reactions that transform harmful pollutants produced during the combustion of fuel into less harmful substances. It is a key component of a vehicle’s exhaust system and plays a critical role in reducing emissions and improving air quality. Here’s how a catalytic converter works:
- Exhaust Gas Flow: The process begins with the flow of exhaust gases from the engine into the catalytic converter through the exhaust pipe.
- Catalyst Activation: Inside the catalytic converter, there are catalysts made of precious metals like platinum, palladium, and rhodium. These catalysts are coated onto a substrate, often a ceramic or metallic honeycomb structure. The catalysts are specifically designed to promote chemical reactions that occur at lower temperatures than would normally be required.
- Oxidation Reactions: As exhaust gases pass through the catalytic converter, they come into contact with the catalysts. In the case of carbon monoxide (CO) and unburned hydrocarbons (HC), the catalysts initiate oxidation reactions. Oxygen from the exhaust combines with these pollutants to form carbon dioxide (CO2) and water (H2O): CO + ½O₂ → CO₂
HC + O₂ → CO₂ + H₂O - Reduction Reactions: Nitrogen oxides (NOx) are targeted through reduction reactions. A different catalyst, often rhodium, promotes the reduction of nitrogen oxides using unburned hydrocarbons present in the exhaust gases. The result is the conversion of nitrogen oxides into nitrogen (N2) and oxygen (O2): NOx + HC → N2 + CO2 + H2O
- Conversion of Pollutants: Through these oxidation and reduction reactions, the harmful pollutants—carbon monoxide, unburned hydrocarbons, and nitrogen oxides—are converted into less harmful substances—carbon dioxide, water, and nitrogen. These products are then expelled from the catalytic converter as exhaust gases.
- Temperature Management: Catalytic converters require a certain operating temperature range to function efficiently. Modern vehicles are equipped with oxygen sensors that monitor the oxygen levels in the exhaust gases. The engine control unit (ECU) uses this information to adjust the air-fuel mixture to maintain the optimal temperature for catalytic converter operation.
In summary, a catalytic converter works by utilizing catalysts to promote oxidation and reduction reactions that convert harmful pollutants into less harmful compounds. This technology significantly reduces the environmental impact of vehicle emissions, contributing to cleaner air and improved air quality.
Types of Catalytic Converters
Certainly, let’s delve into more detail about two-way and three-way catalytic converters:
1. Two-Way Catalytic Converter:
A two-way catalytic converter is a type of emissions control device used in internal combustion engines, primarily gasoline-powered vehicles. It’s called “two-way” because it focuses on targeting two main pollutants—carbon monoxide (CO) and hydrocarbons (HC).
Working Principle:
The primary function of a two-way catalytic converter is to facilitate oxidation reactions that convert carbon monoxide and hydrocarbons into less harmful compounds. It accomplishes this using a catalyst, typically composed of platinum and palladium, which is coated onto a ceramic or metallic substrate. The substrate is often designed in a honeycomb structure to maximize the surface area available for reactions.
When exhaust gases pass through the catalytic converter, they come into contact with the catalyst. The following reactions occur:
- Oxidation of Carbon Monoxide (CO):
CO + ½O₂ → CO₂ Carbon monoxide (CO) reacts with oxygen (O₂) to form carbon dioxide (CO₂), a less harmful greenhouse gas. The platinum and palladium catalyst facilitates this oxidation reaction, promoting the conversion of CO to CO₂. - Oxidation of Hydrocarbons (HC):
HC + O₂ → CO₂ + H₂O Unburned hydrocarbons (HC) in the exhaust gases also undergo oxidation when they react with oxygen, resulting in the formation of carbon dioxide (CO₂) and water (H₂O).
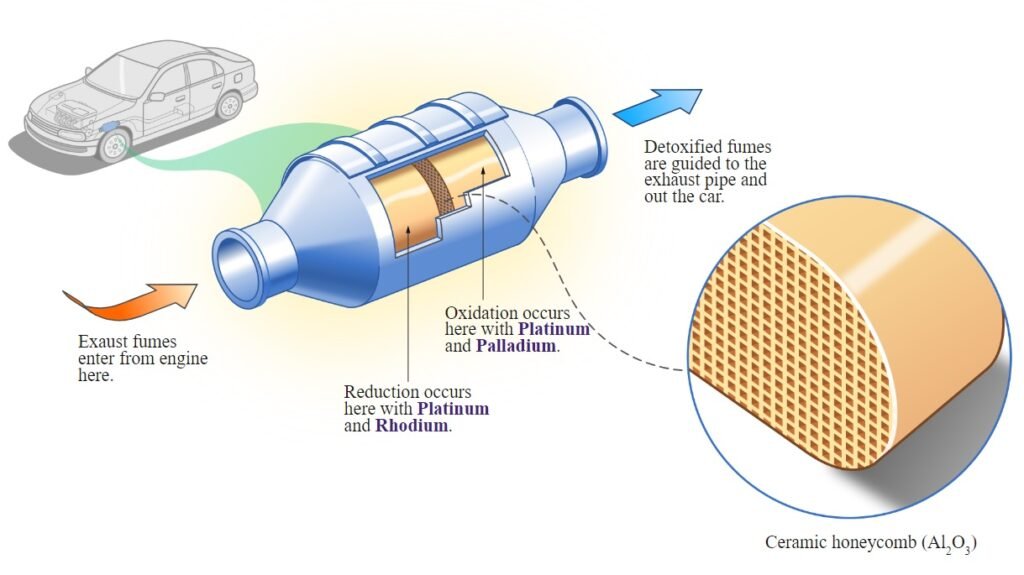
Limitations:
Two-way catalytic converters are effective in reducing CO and HC emissions but do not directly target nitrogen oxides (NOx). They are not as efficient as three-way converters in addressing the full range of pollutants emitted by internal combustion engines. This is why two-way converters are more commonly found in older gasoline-powered vehicles where emission standards were less stringent.
2. Three-Way Catalytic Converter:
A three-way catalytic converter is a more advanced emissions control device widely used in modern gasoline-powered vehicles. It is called “three-way” because it simultaneously addresses three main pollutants—carbon monoxide (CO), hydrocarbons (HC), and nitrogen oxides (NOx).
Working Principle:
A three-way catalytic converter employs a combination of oxidation and reduction reactions to convert the three target pollutants into less harmful compounds. It contains multiple catalysts, typically made of platinum, palladium, and rhodium, which are strategically coated onto the substrate.
- Oxidation of CO and HC: The platinum and palladium catalysts promote the oxidation of carbon monoxide (CO) and hydrocarbons (HC) into carbon dioxide (CO₂) and water (H₂O), as explained in the two-way converter section.
- Reduction of NOx: The rhodium catalyst facilitates the reduction of nitrogen oxides (NOx) by promoting a chemical reaction with the unburned hydrocarbons present in the exhaust gases. This reaction converts nitrogen oxides into nitrogen (N₂) and oxygen (O₂): NOx + HC → N₂ + CO₂ + H₂O
Advantages:
Three-way catalytic converters are highly effective in reducing all three main pollutants—CO, HC, and NOx—in gasoline-powered vehicles. Their ability to simultaneously address these pollutants makes them a crucial component for meeting stringent emissions standards and ensuring cleaner air quality.
In summary, while both two-way and three-way catalytic converters play significant roles in reducing emissions, three-way converters offer a more comprehensive solution by addressing a broader range of pollutants. As emission standards have become more stringent, three-way catalytic converters have become the norm in modern gasoline-powered vehicles due to their efficiency in meeting regulatory requirements and improving air quality.
function of catalytic converter
The primary function of a catalytic converter is to reduce the harmful pollutants and toxic gases produced during the combustion process in internal combustion engines. It achieves this by facilitating chemical reactions that convert these pollutants into less harmful substances before they are released into the atmosphere. The catalytic converter plays a crucial role in improving air quality, reducing environmental impact, and ensuring compliance with emissions regulations. Here’s how it works:
- Oxidation of Carbon Monoxide (CO): Carbon monoxide is a poisonous gas produced by incomplete combustion. The catalytic converter contains catalysts, usually made of platinum and palladium, that promote the oxidation of carbon monoxide into carbon dioxide: CO + ½O₂ → CO₂
- Oxidation of Hydrocarbons (HC): Unburned hydrocarbons are released into the exhaust gases when fuel isn’t fully combusted. The catalysts in the converter promote the oxidation of hydrocarbons into carbon dioxide and water: HC + O₂ → CO₂ + H₂O
- Reduction of Nitrogen Oxides (NOx): Nitrogen oxides are pollutants that contribute to smog and acid rain. The catalytic converter uses a different catalyst, often made of rhodium, to facilitate reduction reactions that convert nitrogen oxides into nitrogen and oxygen: NOx + HC → N₂ + CO₂ + H₂O
The catalytic converter operates within a specific temperature range. It requires heat to activate the catalysts and initiate the chemical reactions. This is why modern vehicles are equipped with oxygen sensors that monitor the exhaust gases and provide feedback to the engine control unit (ECU). The ECU adjusts the air-fuel mixture to maintain the optimal conditions for catalytic converter operation.
In summary, the catalytic converter’s function is to transform harmful carbon monoxide, hydrocarbons, and nitrogen oxides into carbon dioxide, water, and nitrogen, respectively, through oxidation and reduction reactions. This technology significantly reduces the negative impact of vehicle emissions on both the environment and human health.
Signs of Catalytic Converter Issues
Detecting catalytic converter issues early is crucial for maintaining the performance and emissions control of your vehicle. Here are some signs to watch for that might indicate problems with your catalytic converter:
- Check Engine Light: A consistently illuminated check engine light on your dashboard is often the first sign of catalytic converter issues. The vehicle’s onboard diagnostics system detects irregularities in emissions and alerts you through the warning light.
- Reduced Engine Performance: If you notice a decrease in engine power, sluggish acceleration, or poor fuel efficiency, it could indicate a partially clogged or malfunctioning catalytic converter. Restricted exhaust flow can impact engine performance.
- Rattling or Rumbling Noises: A damaged or failing catalytic converter might produce rattling or rumbling noises, especially when the vehicle is idling or when you tap the converter with a rubber mallet. This indicates that the internal substrate might be broken or loose.
- Strong Sulphur Smell: A failing catalytic converter may emit a strong odor of rotten eggs due to sulfur compounds in the exhaust not being properly converted. This smell can be an indicator of incomplete catalytic conversion.
- Excessive Heat: If the catalytic converter is not functioning correctly, it might produce excessive heat, causing the undercarriage or floor of the vehicle to become unusually hot. This can potentially lead to other components being damaged.
- Failed Emissions Test: During vehicle inspections, if your car fails an emissions test due to high levels of CO, HC, or NOx in the exhaust, it could be a sign of a failing catalytic converter.
- Poor Fuel Efficiency: A failing catalytic converter can lead to inefficient combustion and reduced fuel efficiency. If you notice a sudden decrease in miles per gallon (MPG), it’s worth investigating the converter.
- Stalling or Rough Idling: A clogged or malfunctioning catalytic converter might cause the engine to stall or have rough idling, as the exhaust gases are not flowing smoothly.
- Misfires: Misfiring cylinders can lead to unburned fuel entering the exhaust system, potentially damaging the catalytic converter over time.
- Excessive Exhaust Smoke: If you observe blue or black smoke coming from your exhaust pipe, it could be a sign of oil or fuel burning in the exhaust due to an issue with the converter.
If you experience any of these signs, it’s advisable to have your vehicle inspected by a qualified mechanic. Ignoring catalytic converter problems can lead to worsened performance, increased emissions, and potential damage to other engine components. Addressing issues early can help prevent more expensive repairs and ensure your vehicle continues to run efficiently and cleanly.
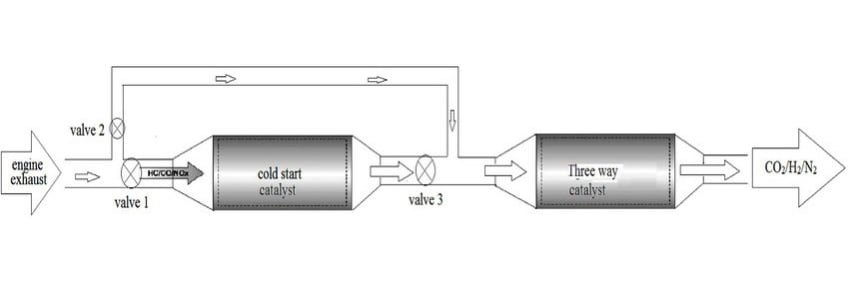
application of catalytic converter
Catalytic converters are widely used in various industries, primarily in the automotive sector, to reduce the emission of harmful pollutants from internal combustion engines. Here are some of the key applications of catalytic converters:
- Automotive Industry: The most common application of catalytic converters is in automobiles, both gasoline-powered and diesel-powered vehicles. They are integrated into the exhaust systems to reduce the emission of pollutants such as carbon monoxide (CO), nitrogen oxides (NOx), and unburned hydrocarbons (HC). This helps vehicles comply with emission regulations and contribute to cleaner air quality.
- Motorcycles and Scooters: Similar to automobiles, catalytic converters are also used in motorcycles and scooters to control exhaust emissions and minimize the environmental impact of two-wheeled vehicles.
- Heavy-Duty Vehicles: Catalytic converters are employed in trucks, buses, and other heavy-duty vehicles to address their significant emission levels. These converters play a crucial role in reducing the release of pollutants into the atmosphere.
- Industrial Applications: Catalytic converters find use in various industrial applications where internal combustion engines are employed, such as power generators and industrial machinery. These converters help mitigate emissions and ensure compliance with environmental regulations.
- Stationary Engines: Engines used for stationary applications, such as backup generators or industrial machinery, can be equipped with catalytic converters to reduce emissions and adhere to emission standards.
- Agricultural Equipment: Farming equipment, such as tractors and harvesters, can incorporate catalytic converters to minimize emissions and promote environmental sustainability in agriculture.
- Construction Machinery: Heavy machinery used in construction and infrastructure projects can be fitted with catalytic converters to decrease emissions and enhance air quality in construction sites.
- Marine Engines: Some marine vessels utilize catalytic converters to control exhaust emissions and reduce the impact of shipping on marine ecosystems.
- Aerospace: While not as common, catalytic converters have been considered for use in aviation to address emissions from aircraft engines, contributing to efforts to reduce the environmental footprint of air travel.
- Power Plants: In certain cases, catalytic converters can be employed in power plants to reduce emissions from fossil fuel combustion and enhance air quality in the surrounding areas.
The widespread adoption of catalytic converters across these various applications underscores their importance in mitigating the environmental impact of combustion processes. By facilitating the conversion of harmful pollutants into less harmful substances, catalytic converters play a vital role in promoting cleaner air and a healthier environment.
advantages of catalytic converter
Catalytic converters offer several important advantages, primarily related to their ability to significantly reduce harmful emissions from internal combustion engines and industrial processes. Here are some key advantages of catalytic converters:
- Emission Reduction: The primary advantage of catalytic converters is their ability to effectively reduce emissions of harmful pollutants, such as nitrogen oxides (NOx), carbon monoxide (CO), and unburned hydrocarbons (HC), from vehicles and industrial sources. This helps improve air quality and reduces the negative health and environmental impacts of these pollutants.
- Environmental Protection: Catalytic converters play a vital role in protecting the environment by helping to minimize the release of pollutants that contribute to smog, acid rain, and greenhouse gas emissions. This contributes to the overall well-being of ecosystems and human populations.
- Regulatory Compliance: Catalytic converters enable vehicles and industrial processes to meet stringent emissions regulations set by governments and environmental agencies. Compliance with these regulations is essential to avoid fines, penalties, and other legal consequences.
- Health Benefits: By reducing the emission of toxic gases and pollutants, catalytic converters contribute to improved public health. Lower levels of pollutants in the air help reduce respiratory illnesses, cardiovascular diseases, and other health issues associated with poor air quality.
- Technological Innovation: The development and implementation of catalytic converter technology have driven advancements in materials science, chemical engineering, and automotive engineering. This innovation has paved the way for more efficient and cleaner combustion processes.
- Improved Fuel Efficiency: Catalytic converters can aid in optimizing engine performance by promoting more complete combustion. This can lead to improved fuel efficiency, as fewer unburned hydrocarbons are wasted, resulting in better mileage for vehicles.
- Versatility: Catalytic converters can be adapted to various types of internal combustion engines, including gasoline, diesel, and alternative fuel engines. This versatility makes them suitable for a wide range of vehicles and industrial applications.
- Reduced Odors: Catalytic converters also contribute to reducing the strong odors associated with exhaust gases from internal combustion engines. This makes the environment more pleasant for both vehicle occupants and bystanders.
- Longevity of Vehicles: By promoting cleaner combustion, catalytic converters can help extend the lifespan of vehicles and engines. Reduced exposure to corrosive and damaging byproducts can lead to less wear and tear on engine components.
- Global Impact: As a widely adopted technology, catalytic converters have made a significant positive impact on a global scale. They have contributed to reducing the overall carbon footprint of transportation and industrial activities.
In summary, catalytic converters offer a multitude of advantages, ranging from environmental protection and public health benefits to regulatory compliance and technological progress. Their role in mitigating the negative effects of emissions makes them a crucial component in modern vehicles and industrial processes.
disadvantages of catalytic converter
Certainly, here are the potential disadvantages of catalytic converters summarized in bullet points:
- Cost: Catalytic converters can be expensive to manufacture and replace. The use of precious metals like platinum, palladium, and rhodium in the catalyst increases production costs.
- Limited Efficiency at Low Temperatures: Catalytic converters require a certain temperature to operate efficiently. During cold starts, they may not reach optimal efficiency immediately, leading to higher emissions during those moments.
- Catalyst Deactivation: Over time, catalysts can become deactivated or damaged due to factors like exposure to leaded gasoline, engine misfires, or contamination from certain chemical compounds.
- Backpressure: The design of catalytic converters can introduce backpressure into the exhaust system, potentially reducing engine performance and fuel efficiency.
- Impact on Performance: In high-performance engines, catalytic converters can slightly restrict exhaust flow, affecting power output. Some enthusiasts opt for aftermarket modifications that remove or replace catalytic converters.
- Maintenance and Replacement: If a catalytic converter fails, it may need to be replaced, which can be costly. Regular maintenance is necessary to prevent issues and ensure proper functioning.
- Recycling Challenges: Recycling catalytic converters to reclaim precious metals can be complex and resource-intensive due to the heterogeneous nature of the components.
- Precious Metal Theft: The valuable metals present in catalytic converters make them a target for theft, especially in areas with high metal prices.
- Environmental Impact of Mining Precious Metals: The mining and extraction of precious metals used in catalytic converters can have environmental consequences, including habitat disruption and pollution.
- Complexity of Repairs: Repairing or replacing catalytic converters often requires specialized knowledge and equipment, which can make repairs more complex and costly.
Despite these disadvantages, the overall benefits of catalytic converters in terms of emissions reduction and environmental protection usually outweigh these drawbacks.
Check Out Other Important Topics
| IC Engine | Important PDFs | Boilers | Synergy Maritime Exam | Naval Arch | MEO Class 4 |
| Interview Questions | Difference Between | Types of Pumps | Auxiliary Machines | Types of Valves | Home |