In this article we will learn about what are the different methods of steel making process steps and the Flowchart to understand better. There are different processes involved according to the type and composition of the steel. Lets have a look on the various process of making steel.
Steel is fundamentally an alloy of iron and carbon, with the carbon content varying up to 1.5 per cent. The carbon is distributed throughout the mass of the metal, not as elemental or free carbon but as a compound (chemical combination) with iron.
If however, the carbon is increased above 1.5 per cent, a stage soon arrives when no more carbon can be contained in the combined state and any excess must be present as free carbon (graphite). It is at this stage that the metal merges into the group termed cast iron. Therefore, for a material to be classed as steel there must be no free carbon in its composition ; immediately free graphite that occurs passes into the category of cast iron.
Besides carbon, there are other elements present in the steel, e.g., Sulphur, silicon, phosphorus, manganese, etc.; but carbon is by far the most important modifying element. Iron forms the mass of the alloy ; it is the quantity partner while on carbon falls the duty of determining the quality of the steel to meet demand which iron alone cannot satisfy. The importance of carbon in steel lies not in its relative volume but in its remarkable influence on the internal structural changes and subsequently cooled by various methods.
Steel Making Process
The commercial processes for making steel are :
(1) Bessemer process (2) L-D process (3) Open-hearth process (4) Crucible process (5) Electric process (6) Duplex process.
The Bessemer, open hearth and electric process can be subdivided into (a) acid process and (b) basic process, according to the type of lining used in the furnace. In each of these processes, the steel is produced either by adding carbon to wrought iron or by removing the proper portion of carbon from pig iron by first completely decarburising pig iron, and then adding the proper amount of carbon. Let’s understand the Steel making process steps in detail.
1. The Bessemer Process
The Bessemer steel making process consists of blowing air through molten pig iron contained in a special furnace known as a converter which shaped like a huge concrete mixer (Fig. 4.2). The converter is made of steel plates lined inside with a refractory material. The type of refractory lining used depends upon the character of the steel-making process, i.e., upon the acid process or basic process.
In the acid process, the converter is lined with silica brick which is known in the refractory trade as “acid”. The acid process does not eliminate phosphorus or Sulphur from the metal. In the basic process, the converter is lined with dolomite, which is known as “basic”. It removes phosphorus and to some extent Sulphur.
2. The L-D Process
The latest development in steel making process is the name of this process which comes from the initials of two separate plants in Austria, at Linz and Donawitz. The local Austrian ore is too low in phosphorus to enable the air-blown basic Bessemer method to be used. Since air, a mixture of nitrogen and oxygen, is used, the resulting steel contains nitrogen which makes steels liable to brittleness under certain conditions.
Further the bulk of the nitrogen which is not dissolved carries away so much heat that only a metal of high phosphorus content will generate enough heat to give the required temperature of the liquid steel. The remedy has been to replace the air blast by oxygen or a gas mixture containing no nitrogen.
3. The Open Hearth Process
In the open hearth process for producing steel, pig iron, steel scrap, and iron oxide in the form of iron ore or scale are melted in a Siemens-Martin open hearth furnace (Fig. 4.4), so called because the molten metal lies in a comparative shallow pool on the furnace bottom or hearth. The hearth is surrounded by a roof and walls of refractory bricks. The charge is fed through a charging door and heated to 1,600°C to 1,650°C mainly by radiation of heat from the burning of gaseous fuels above it. It is not the amount of heat but rather the high temperature heat that is essential for the purpose.
4. The Crucible Steel Making Process
In the crucible steel making process , mixtures of wrought iron, steel scrap and ferromanganese are melted down with charcoal in an air tight crucible. Other ferro alloys may be added when alloy steel is produced by this process. In the crucible process, carbon is added to the iron as the carbon content of wrought iron is low.
Necessary carbon is taken up by the metal from the charcoal during melting. After the materials are melted and thoroughly alloyed, the crucibles are taken from the furnace known as regenerative furnace which is heated by a gaseous fuel as in the open hearth furnace, and finally the steel is poured into mould. The length of time between placing the crucible in the furnace and withdrawing the crucible is approximately four hours.
The process is primarily a melting and alloying process and no refining of the metal takes place in the crucible.
5. The Electric Process
Within recent years, the melting of steel by electric furnaces has developed rapidly for the availability of cheap electric power. Electricity is used solely for the production of heat and does not impart any special properties to the steel. Nevertheless, the electric furnace has the following advantageous features.
1. It generates extremely high temperature, about 2,000°C , in the melting chamber without introducing oxygen or nitrogen from the air or impurities from the fuel. This facilitates the removal of the harmful impurities such as oxygen, Sulphur and phosphorus, and also nonmetallic inclusions.
2. The temperature at all times may be easily controlled and regulated
3. It permits the addition of expensive alloying elements such as chromium, nickel, tungsten, etc. without loss by oxidation.
4. A great variety of steels, differing in carbon content and with any content of alloying elements, can be manufactured.
6. Duplex Processes
Combination methods of steel making process known as duplex processes are carried out in two steel making units. The following combinations are usually done.
1. Basic and acid open-hearth
2. A basic open-hearth furnace and a basic electric furnace.
3. A Bessemer converter and a basic open hearth furnace.
Steel Making Process Flowchart


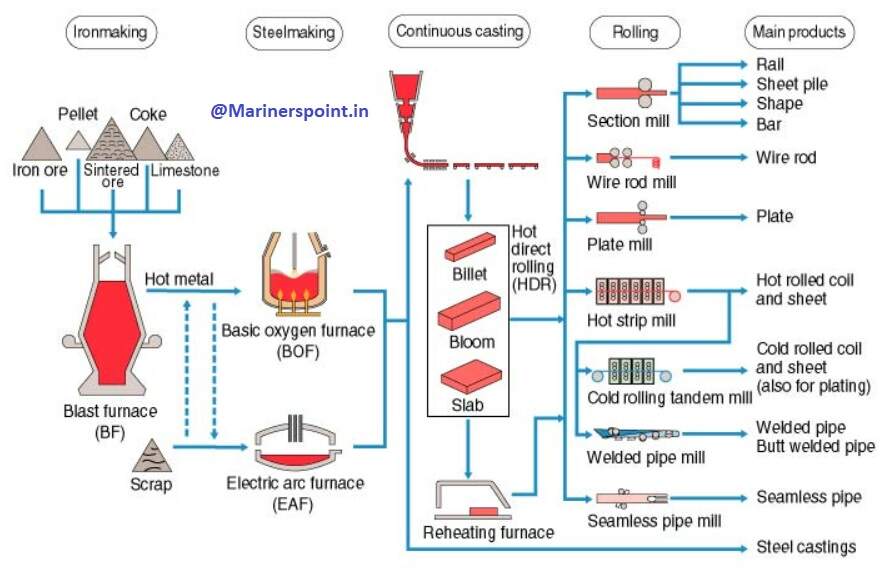
Frequently Asked Questions
What is steel process?
Steel is typically produced in two stages. The basic oxygen furnace (BOF) process or melting scrap steel or direct reduced iron (DRI) in an electric arc furnace are used to convert liquid iron into steel in the main steelmaking stage. Secondary steelmaking is a refining process that involves the addition of alloying metals and the removal of impurities.
Which gas is used for manufacture of steel?
Industrial gases usually used in steel industry are oxygen, nitrogen, argon and hydrogen.
What did the steel process do?
The Bessemer steel making process consists of blowing air through molten pig iron contained in a special furnace known as a converter which shaped like a huge concrete mixer.
Who made the steel process?
Sir Henry Bessemer
How is carbon added to steel?
Iron ore, coke (made from coal), and lime are used to make virgin steel in a blast furnace. The raw materials are placed on top of the furnace, which has a temperature of 3000 degrees Fahrenheit. Carbon is released into the molten product as the iron ore melts and interacts with the burning coke.
These were the different types of Steel Making Process steps in detail including the flowchart. We have covered all types of steel making process and tried to explain with proper diagrams and images wherever required. Hope you liked this article. Please give your reviews in the comments below.
Check Out Other Important Topics
Different Types of Steel & Constituents of Steel
Cast Iron – Properties, Types & Uses
Recovery Recrystallization and Grain Growth – Working Process
Constrained Motion – Definition, Types, Examples, Images
| IC Engine | Important PDFs | Boilers | Synergy Maritime Exam | Naval Arch | MEO Class 4 |
| Interview Questions | Difference Between | Types of Pumps | Auxiliary Machines | Types of Valves | Home |



Very well explained the steel making process.